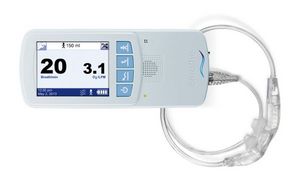
IRVINE, CA--(Marketwired - Oct 23, 2013) - Breathe Technologies, the developer and manufacturer of innovative medical technologies to treat lung diseases, announced that two presentations at the CHEST 2013 meeting in Chicago, October 26-31 will highlight research conducted using the company's NIOV™ System. These presentations come on the heels of a recent publication in the American Journal of Respiratory Critical Care Medicine (AJRCCM), which demonstrated the NIOV System's effectiveness in providing dramatic improvements in shortness of breath and exercise endurance for patients with Chronic Obstructive Pulmonary Disease (COPD). The NIOV System is a 1-lb, wearable, ambulatory ventilation system for the treatment of symptoms associated with variety of respiratory insufficiency diseases.
The two presentations at CHEST highlighting the NIOV System are as follows:
- An oral presentation, "Effect of the Breathe Noninvasive Open Ventilation (NIOV) Device on Breathing Pattern of Patients with Severe COPD" will be delivered by Matthew Cohn, M.D., Clinical Associate, Tufts University School of Medicine, during the Dyspnea in COPD session, on Sunday October 27, 2013. This presentation highlights research conducted in conjunction with Nicholas Hill, M.D., Professor of Medicine; Chief, Pulmonary, Critical Care, and Sleep Division, Department of Medicine, Tufts University School of Medicine.
- A poster presentation titled "Effects of Noninvasive Open Ventilator on the Tidal Volume and Inspiratory Effort During Exercise in Severe COPD" will be delivered by Robert Cao, M.D., Research Associate, Los Angeles Biomedical Research Institute at Harbor-UCLA Medical Center, during the COPD Treatment Poster session, on Wednesday, October 30, 2013.
Dr. Cao's presentation will be a new data analysis based upon his recently co-authored study "Physiologic Effects of an Ambulatory Ventilation System in Chronic Obstructive Pulmonary Disease" published in AJRCCM. Dr. Cao co-authored the study with Richard Casaburi, M.D. M. Eng., Ph.D. Professor of Medicine, Associate Chief of Research, Division of Respiratory and Critical Care Physiology, UCLA David Geffen School of Medicine and Janos Porszasz, M.D., Ph.D., Adjunct Professor of Medicine, Technical Director, Rehabilitation Clinical Trials Center, Los Angeles Biomedical Institute at Harbor-UCLA Medical Center. The study demonstrated the effectiveness of the NIOV System in the treating the symptoms associated with advanced stage COPD, including shortness of breath and exercise endurance. The AJRCCM study compared the NIOV System to the current standard of care, long term oxygen therapy (LTOT), and demonstrated the following:
- 54% increase in exercise endurance
- 28% reduction in Borg Dyspnea Scale, a measurement of shortness of breath
- 46% reduction in accessory respiratory muscle activation
- Increased and maintained oxygen saturation levels during exercise from 92.7% to 98.5%
"Our study indicates that the NIOV System dramatically prolongs exercise tolerance. The magnitude of this improvement is substantially greater than what we have observed with either bronchodilator or routine oxygen therapy. It is similar to the improvements seen with pulmonary rehabilitation," said Dr. Casaburi.
Patients living with COPD may be limited in their ability to participate in activities of daily living and exercise due to the symptoms associated with their disease. The NIOV System was designed to address these symptoms and facilitate ambulation. Research, such as the recent study in the AJRCCM, demonstrates the clinical efficacy of the NIOV System in reducing dyspnea, unloading respiratory muscles and improving exercise endurance.
"We believe the NIOV System may become a new standard of care for the treatment of patients with respiratory insufficiency," said Larry Mastrovich, president and CEO of Breathe Technologies, Inc. "NIOV has the potential to transform COPD patients' quality of life by reducing shortness of breath and increasing exercise endurance, allowing them to lead more active and independent lives."
About the NIOV System
The NIOV System is a 1-lb, wearable, ambulatory ventilation system for the treatment of symptoms associated with a variety of respiratory insufficiency diseases. The NIOV System increases tidal volume by providing positive pressure ventilation that has been shown1 to significantly:
- Improve ventilation
- Reduce dyspnea
- Increase oxygenation
- Significantly enhance exercise endurance
- Unload respiratory muscle activity
The NIOV System is designed for applications such as supporting activities of daily living, patient ambulation, physical therapy, and pulmonary rehabilitation in either home or institutional environments.
About Breathe Technologies, Inc.
Breathe Technologies, Inc. is a developer and manufacturer of innovative medical technologies that have the potential to dramatically improve quality of life in the treatment of lung diseases. The company's first product, the NIOV™ System, is cleared by the FDA for use in both the institutional and homecare settings. Breathe's pipeline includes a discreet CPAP device for sleep apnea to improve user experience and address the unmet need in this market. Investors include Kleiner Perkins Caufield & Byers, Johnson & Johnson Development Corporation, Delphi Ventures, DAG Ventures, Morgan Creek, and Synergy Partners International. Breathe is located in Irvine, CA. For more information, please visit www.breathetechnologies.com.
1 Porszasz J, et al. Physiologic effects of an ambulatory ventilation system in COPD. Am J Respir Crit Care Med Vol 188, Iss. 3, pp 334-342, Aug 1, 2013
Contact Information:
Media Contacts:
Stephanie Marks for Breathe Technologies
smarks@lazarpartners.com
212-843-0211
Brian Baxter for Breathe Technologies
bbaxter@lazarpartners.com
646-871-8491
Company Contact:
Gary Berman
Vice President, Corporate Development & Finance
GaryB@breathetechnologies.com
949-988 -7755