Ottawa, May 06, 2024 (GLOBE NEWSWIRE) -- The global retinitis pigmentosa market size was valued at USD 14.02 billion in 2023 and is predicted to hit around USD 19.90 billion by 2032, a study published by Towards Healthcare a sister firm of Precedence Research.

Insight Highlights:
- Autosomal Recessive RP led the way in 2023, accounting for a significant 65% market share.
- But excitement surrounds gene therapy, holding a promising 23% share and offering hope for countless patients.
- While North America currently holds the largest share, Asia Pacific is sprinting ahead with the fastest growth, potentially changing the landscape.
Download a short version of this report @ https://www.towardshealthcare.com/personalized-scope/5114
In 2022, approximately 82,500 to 110,000 individuals in the United States were affected by retinitis pigmentosa. This underscores the growing demand for innovative approaches to address the condition, such as gene therapy and retinal implants.
Retinitis Pigmentosa comprises genetic eye disorders impacting the retina, the eye's vision center. It results in the deterioration of photoreceptor cells crucial for vision in various light conditions and clarity. As it progresses, individuals may encounter night or low-light vision challenges, reduced peripheral vision, and potentially total blindness.
Retinitis Pigmentosa is divided into two main types: non-syndromic and syndromic. Non-syndromic retinitis pigmentosa, which makes up 70-80% of cases, doesn't involve other systemic abnormalities. Syndromic retinitis pigmentosa, accounting for 20-30% of cases, is associated with additional non-ocular syndromes and systemic diseases. Non-syndromic retinitis pigmentosa is linked to mutations in 80 different genes, with around 20% being autosomal recessive, 10-20% autosomal dominant (AD), and 10% X-linked recessive.
For instance,
- In 2021, according to the National Institute of Health, a study conducted in Spain, which represents the largest group of patients with inherited retinal diseases reported globally, aligns with these estimates. Previously, it was believed that only males were affected by X-linked Retinitis Pigmentosa (XLRP). Still, it has been observed that female carriers can display various symptoms, ranging from no symptoms to severe retinal disease. The remaining cases of retinitis pigmentosa are considered "sporadic," meaning there is no identified inheritance pattern or molecular mechanism.
You can place an order or ask any questions, please feel free to contact us at sales@towardshealthcare.com
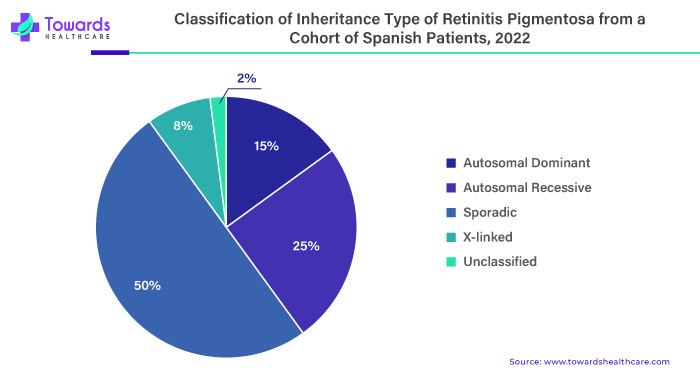
The importance of understanding and addressing retinitis pigmentosa lies in its impact on individuals' quality of life. Vision loss can significantly affect a person's independence, mobility, and overall well-being. Additionally, retinitis pigmentosa is often hereditary, meaning it can affect multiple generations within a family. Advancements in research, diagnosis, and treatment options for retinitis pigmentosa are essential for improving outcomes and providing hope for affected individuals and their families.
In 2023, the CDC revealed that around 12 million Americans aged 40 and above experience vision impairment, including 1 million who are blind, 3 million with correctable vision issues, and 8 million due to untreated refractive errors. As the global population ages and awareness of genetic diseases rises, there's a growing demand for retinitis pigmentosa treatments and technologies. Precision medicine and gene therapy are gaining traction for addressing genetic disorders like retinitis pigmentosa. Furthermore, advancements in gene editing, stem cell therapy, and retinal implants offer promising avenues for managing retinitis pigmentosa and enhancing visual function. Increased investment in research and development by biotech and pharmaceutical firms, academic institutions, and government bodies bolsters the retinitis pigmentosa treatment market. Moreover, regulatory agencies are streamlining approval processes for innovative therapies, potentially expediting the availability of new retinitis pigmentosa treatments. The market for retinitis pigmentosa treatments and technologies is expected to grow steadily as scientific understanding improves, new therapeutic approaches emerge, and the demand for solutions to address vision loss continues to rise.
Newer Techniques Help in Early Detection of Retinitis Pigmentosa
Early detection and diagnosis of retinitis pigmentosa are super important because they help catch the condition early, allowing doctors to start the right treatments sooner. Techniques like genetic testing and eye exams can spot Retinitis Pigmentosa before it worsens, giving people a better chance at managing it. Vision rehab is also crucial. It helps folks with retinitis pigmentosa learn new ways to navigate the world with their changing vision, using tools like special glasses or learning mobility skills. Genetic counseling is essential for families affected by retinitis pigmentosa. It helps them understand how the condition can be passed down and make intelligent choices about family planning. Research is ongoing to find new treatments for retinitis pigmentosa, like gene therapy or stem cell therapy, which could slow or even reverse the damage. Additionally, psychosocial support is crucial for people living with retinitis pigmentosa. It offers them emotional support, counseling, and connections with others who understand what they're going through, helping them cope better with the challenges of vision loss.
Customize this study as per your requirement @ https://www.towardshealthcare.com/customization/5114
Increasing Prevalence of Retinitis Pigmentosa Patients
In 2022, the National Institute of Health estimated that retinitis pigmentosa is the most common inherited retinal disorder, affecting over 1.5 million people worldwide. The rise in retinitis pigmentosa cases globally is mainly due to two reasons: more people getting older and an increase in genetic disorders. People living longer are more likely to develop retinitis pigmentosa, often appearing later in life. Since retinitis pigmentosa is mainly passed down through families, better genetic testing and awareness have identified more cases. Also, when relatives intermarry, it can add to the number of retinitis pigmentosa cases. With more people being diagnosed with retinitis pigmentosa, there's a greater need for effective treatments. This growing need opens up opportunities for research to find new therapies and improve existing ones. It's essential for healthcare and research to keep working on understanding retinitis pigmentosa and finding better ways to manage it as the number of affected individuals continues to grow.
Gene Therapy has Become a Hopeful Treatment Method for Retinitis Pigmentosa
Gene therapy is used in the treatment approach for Retinitis Pigmentosa, aiming to address the underlying genetic mutations responsible for the condition. Retinitis Pigmentosa encompasses diverse inherited retinal disorders, many caused by mutations in specific genes essential for normal retinal function. Gene therapy seeks to correct the genetic defects underlying RP and restore vision by delivering functional copies of these genes into retinal cells.
Recent News:
- Spark Therapeutics' Luxturna, a gene therapy designed for RPE65-associated Retinitis Pigmentosa, obtained FDA approval for marketing in 2022, marking the first-ever approval of a gene therapy for an inherited condition in the United States.
Gene therapy for Retinitis Pigmentosa typically involves using viral vectors or other delivery systems to transport therapeutic genes into the retina. Viral vectors, such as adeno-associated viruses (AAVs), are often employed because they efficiently deliver genes to target cells without causing harmful immune responses. Once introduced into the retina, the therapeutic genes integrate into the retinal cells and produce functional proteins necessary for proper visual function.
Clinical trials investigating gene therapy for retinitis pigmentosa have shown promising results, with some patients experiencing improved vision following treatment. These improvements may include slowed disease progression, preservation of remaining vision, or, in some cases, partial restoration of lost vision. Gene therapy holds particular promise as a targeted treatment approach for individuals with specific genetic mutations associated with retinitis pigmentosa.
For instance,
- In 2023, Coave, a French biotech firm, is conducting a Phase 1/2 trial for RP linked to mutations in the PDE6B gene. This study will involve enrolling 12 patients and is anticipated to span three years.
- In 2021, various companies were at different stages of clinical trials for gene therapy to treat X-linked retinitis pigmentosa (XLRP), a specific type of RP caused by mutations in the RPGR gene. Johnson & Johnson is in Phase 3, Beacon is in Phase 2, and 4DMT is in Phase 1/2. Initial findings indicate promising enhancements in vision, including retinal sensitivity and visual acuity.
As gene therapy techniques continue to be refined and optimized, there is growing optimism regarding their potential to slow or halt the progression of retinitis pigmentosa in affected individuals. Ongoing research efforts aim to improve gene therapy's safety, efficacy, and delivery methods for retinitis pigmentosa, further enhancing its therapeutic potential.
Due to the promising results observed in clinical trials and the increasing understanding of the genetic basis of retinitis pigmentosa, the market for gene therapy in retinitis pigmentosa treatment has experienced significant growth. Pharmaceutical companies, biotechnology firms, and research institutions are investing resources into developing and commercializing gene therapy products for retinitis pigmentosa, driving market expansion. Additionally, regulatory agencies have shown interest in expediting the approval process for gene therapy treatments for rare genetic disorders like retinitis pigmentosa, further contributing to market growth.
Gene therapy represents a groundbreaking approach to treating retinitis pigmentosa by targeting the underlying genetic defects responsible for the condition. As gene therapy techniques advance, they offer hope for improving outcomes and quality of life for individuals affected by retinitis pigmentosa, leading to increased market demand and investment in retinitis pigmentosa treatment options.
With Retinal Implants, People Can See Again
Retinal implants, also known as artificial retinas or bionic eyes, represent an innovative technology designed to restore vision in individuals with severe vision loss, such as those affected by Retinitis Pigmentosa. These innovative devices electrically stimulate the remaining retinal cells to generate visual perceptions, bypassing the retina's damaged or non-functioning photoreceptor cells.
For instance,
- On September 6, 2021, Cirtec Medical, a U.S.-based medical technology company, partnered with Bionic Vision Technologies (BVT) in a strategic partnership. Cirtec gained a significant share in BVT through this deal, which is expected to help enhance BVT's new retinal implants.
- In 2020, Pixium Vision started developing a device called the Bionic Iris. It uses tiny solar cells placed in the iris to provide power for stimulating the retina. Early tests show hopeful outcomes, but the device is still being researched.
The essential components of retinal implants typically include an electrode array implanted on the retina and an external camera system. The camera system captures visual information from the surrounding environment and processes it into electrical signals transmitted to the electrode array. The electrode array stimulates the remaining retinal cells, such as ganglion cells, which transmit visual information to the brain, thus generating visual perceptions.
While current retinal implants have demonstrated the ability to restore some degree of vision in individuals with severe vision loss, they still have limitations regarding visual acuity and resolution. Ongoing research efforts aim to overcome these limitations and enhance the performance and functionality of retinal implants. One area of focus in retinal prosthesis research is improving electrode design to achieve more precise and targeted stimulation of retinal cells. Researchers aim to enhance electrical stimulation's specificity and effectiveness by optimizing electrode materials, shapes, and configurations, thereby improving user visual outcomes.
Additionally, advancements in surgical techniques are crucial in retinal implants' successful implantation and functioning. Minimally invasive surgical approaches, precise electrode array placement, and tissue integration improvements are essential for maximizing retinal implant device's long-term efficacy and safety.
Ongoing research aims to expand the applicability of retinal prostheses to a broader range of retinitis pigmentosa patients, including those with varying degrees of retinal degeneration. By addressing current limitations and improving performance and functionality, retinal implants hold tremendous promise for significantly improving the quality of life for individuals with severe vision loss due to conditions like retinitis pigmentosa.
Browse More Insights of Towards Healthcare:
- The global brain computer interface market size was valued at USD 2,130 million in 2022 and is estimated at USD 9,445.1 million by 2032, growing at a healthy 16.7% CAGR from 2023 to 2032.
- The global single-use bioprocessing market revenue surpassed USD 20.96 billion in 2022 to reach around USD 84.14 billion by 2032, at a double-digit CAGR of 15.46% from 2023 to 2032.
- The global liquid biopsy market is estimated to grow from USD 4,722.73 million in 2022 to reach around USD 18,280.13 million by 2032, expanding at a CAGR of 14.5% between 2023 and 2032.
- The global AI in medical imaging market size to grow from USD 762.84 million in 2022 and is projected USD 14,423.15 million by 2032, at a healthy 34.8% of CAGR between 2023 and 2032.
- The global CAR T-Cell therapy market is estimated to grow from USD 3.87 billion in 2022 to reach around USD 88.52 billion by 2032, staggering at 29.8% of CAGR between 2023 and 2032.
- The Europe cell culture media market is estimated to grow from USD 771.2 million in 2022 to reach an around of USD 1,453.2 million by 2032, growing at a 6.29% CAGR between 2023 and 2032.
- The global insulin pump market was valued at USD 5.71 billion in 2022 and is projected to hit USD 15.28 billion by 2032, expanding at a CAGR of 9.65% from 2023 to 2032.
- The global behavioral rehabilitation market size accounted for USD 154.67 billion in 2022 to surpass around USD 221.33 billion by 2032, expanding at a CAGR of 3.58% from 2023 to 2032.
- The cell and gene therapy manufacturing market size was valued at USD 3,755.4 million in 2022 to reach around of USD 13,603.4 million by 2032, at a double-digit CAGR of 16.25% from 2023 to 2032.
- The global AI in magnetic imaging (MRI) market size was estimated at USD 5.77 billion in 2022 and is expected to hit around USD 10.8 billion by 2032, growing at a CAGR of 6.23% from 2023 to 2032.
Impact of Expensive Procedures
Developing new therapies for retinitis pigmentosa, such as gene therapy and stem cell therapy, is multifaceted and costly. The process begins with extensive primary research to uncover the underlying causes of retinitis pigmentosa and identify potential therapeutic targets. This phase involves conducting experiments in laboratory settings, which require specialized equipment, reagents, and expertise. Following this, preclinical studies evaluate potential therapies' safety, efficacy, and pharmacokinetics using animal retinitis pigmentosa models. These studies are crucial for understanding how the treatments may work before moving on to human trials.
In 2023, the World Health Organization (WHO) stated that vision problems create a significant financial strain worldwide, with the yearly global productivity cost estimated at USD 411 billion. Rising treatment expenses present challenges in the Retinitis Pigmentosa market. Clinical trials represent the final testing phase, involving multiple stages (Phase I to Phase III) to assess the therapies' safety, dosage, and efficacy in human participants. Clinical trials are highly regulated, requiring careful planning, coordination, and compliance with stringent regulatory standards set by agencies such as the Food and Drug Administration (FDA). Meeting these standards entails additional costs and complexities, including securing regulatory approvals, recruiting and retaining participants, and managing trial data.
As therapies progress through clinical development, efforts are made to scale up production for potential commercialization. This requires establishing robust manufacturing processes and facilities to ensure product consistency, quality, and compliance with Good Manufacturing Practice (GMP) standards. Additionally, protecting intellectual property through patents is essential for securing market exclusivity and attracting investment in retinitis pigmentosa therapies. However, obtaining and maintaining patents can be expensive and time-consuming.
Small biotechnology companies and academic researchers often need help securing the necessary funding to support the development process. They rely on various funding sources, including government grants, private investments, collaborations with larger companies, and philanthropic support, to advance retinitis pigmentosa treatments from the laboratory to clinical use. Despite the obstacles, continued investment and cooperation are essential for driving progress in developing effective therapies for RP and improving outcomes for individuals affected by this debilitating condition.
Geographical Landscape
North America, particularly the United States, is a significant hub for retinitis pigmentosa research, development, and market activity. The region boasts leading academic institutions, research centers, and biotechnology companies focused on ophthalmic research and innovative therapies for retinitis pigmentosa. Additionally, the U.S. Food and Drug Administration (FDA) is central in regulating RP treatments and approving new therapies for commercialization.
The geographic landscape of the retinitis pigmentosa market in Europe encompasses various regions with differing prevalence levels, access to healthcare, and regulatory frameworks. Countries such as the United Kingdom, Germany, France, Italy, and Spain are prominent markets for retinitis pigmentosa treatment and research in Europe due to their well-established healthcare systems and significant patient populations.

The Asia-Pacific region is emerging as a growing market for retinitis pigmentosa treatments, fueled by advancements in healthcare infrastructure, increasing awareness of genetic disorders, and rising healthcare expenditure. Countries like Japan, China, and South Korea invest in ophthalmic research and development while serving as essential markets for retinitis pigmentosa therapies.
Competitive Landscape
The competitive landscape of the Retinitis Pigmentosa market encompasses a range of stakeholders, including pharmaceutical companies, biotechnology firms, academic institutions, research organizations, and healthcare providers. These entities are engaged in various activities, such as research and development of novel therapies, manufacturing and commercialization of existing treatments, and provision of healthcare services to retinitis pigmentosa patients. Government agencies, such as the National Institutes of Health (NIH) in the United States and the European Commission's Horizon 2020 program, provide funding and support for research and development initiatives for retinitis pigmentosa. These agencies play a crucial role in fostering innovation, facilitating collaborations, and advancing therapeutic discoveries in the field of ophthalmology.
Recent Developments
- In January 2022, 4D Molecular Therapeutics said the FDA gave special status to 4D-125, a treatment for X-linked retinitis pigmentosa. This treatment aims to deliver a working version of the RPGR gene to the retina's light-sensitive cells. It's made to be easily given into the eye and was developed by 4D Molecular Therapeutics.
- In 2022, Bionic Vision Technologies (BVT) announced that their Bionic Eye System in Australia has been granted Breakthrough Device designation by the FDA in the United States, representing a significant advancement in bionic vision technology.
- In 2022, a patient in the Netherlands underwent a procedure to receive the Prima System, a bionic eye implant created by Pixium Vision in France. This implant provides optimism for individuals with geographic atrophy (GA), the most common type of dry age-related macular degeneration (AMD), by offering potential restoration of partial vision.
Market Players
- Novartis
- Roche (Spark Therapeutics Inc)
- MeiraDx
- Astellas Pharma Inc.
- Clino Corporation
- Gensight Biologics
- InFlectis BioScience
- Bausch Health Companies Inc.
- Adverum Biotechnologies
- ReGenx Biosciences
Market Segments
By Disease Type
- Autosomal Dominant RP
- Autosomal Recessive RP
- X-linked RP
By Treatment Type
- Vit A Supplements
- Gene Therapy
- Vit A Supplements
- Gene Therapy
- Retinal Implants
- Low Vision Aids
- Surgery
- Others
By Geography
- North America
- Europe
- Asia Pacific
- Latin America
- Middle East and Africa
Acquire our comprehensive analysis today @ https://www.towardshealthcare.com/price/5114
You can place an order or ask any questions, please feel free to contact us at sales@towardshealthcare.com
Gain access to the latest insights and statistics in the healthcare industry by subscribing to our Annual Membership. Stay updated on healthcare industry segmentation with detailed reports, market trends, and expert analysis tailored to your needs. Stay ahead of the curve with valuable resources and strategic recommendations. Join today to unlock a wealth of knowledge and opportunities in the dynamic world of healthcare: Get a Subscription
About Us
Towards Healthcare is a leading global provider of technological solutions, clinical research services, and advanced analytics to the healthcare sector, committed to forming creative connections that result in actionable insights and creative innovations. We are a global strategy consulting firm that assists business leaders in gaining a competitive edge and accelerating growth. We are a provider of technological solutions, clinical research services, and advanced analytics to the healthcare sector, committed to forming creative connections that result in actionable insights and creative innovations.
Browse our Brand-New Journals:
https://www.towardspackaging.com
https://www.towardsautomotive.com
Web: https://www.precedenceresearch.com
For Latest Update Follow Us: https://www.linkedin.com/company/towards-healthcare
Get Our Freshly Printed Chronicle: https://www.healthcarewebwire.com
